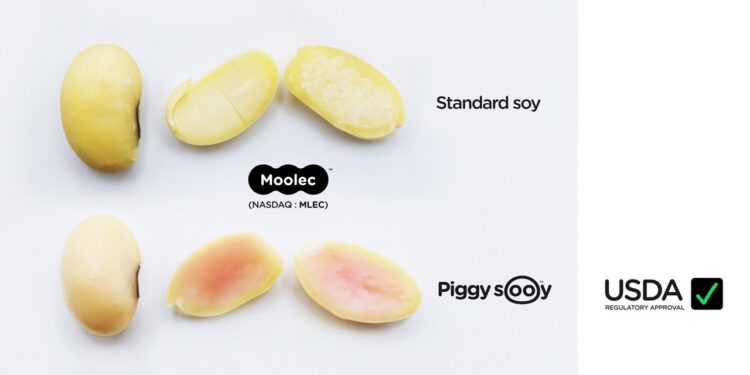
Moolec Science, a molecular farming food-ingredient company, has announced that the Animal and Plant Health Inspection Service (APHIS) of the US Department of Agriculture (USDA) has concluded its Regulatory Status Review (RSR) for Moolec’s genetically engineered (GE) soybean ‘Piggy Sooy’.
The USDA-APHIS RSR determines that Moolec’s genetically engineered soybean, accumulating animal meat protein, is unlikely to pose an increased plant pest risk relative to non-engineered soybeans. Therefore, it is not subject to the APHIS regulation that governs the movement of organisms modified or produced through genetic engineering (as described in 7 CFR part 340).
“Moolec embraced Nasdaq’s slogan ‘Rewrite Tomorrow’ and took it literally! We achieved an unprecedented milestone in biotechnology with the first-ever USDA-APHIS approval of this kind,” commented Gaston Paladini, Moolec Science’s CEO & Co-Founder. “We are unlocking the power of plants by leveraging science to overcome climate change and global food security concerns. I am very proud of the Moolec team, creating value for shareholders and the planet at the same time.”
This milestone reinforces Moolec’s B2B go-to-market strategy for Piggy Sooy product, an innovative, functional, and nutritious ingredient. By adding a well-known animal meat protein (porcine myoglobin) to the standard soybean proteins, the company expects to provide food manufacturers with a unique ingredient that will have a positive carbon and water footprint.
“We believe this milestone sets the stage for a revolution in the food-industrial biotech landscape, paving the way for expedited adoption of molecular farming technology by other industry players,” added Martin Salinas, Chief of Technology & Co-Founder at Moolec. “Also, this compelling advancement signifies a stride in enhancing our operational efficiency, transforming our methods of raw material sourcing, and optimizing our downstream crushing and processing operations.”
In June 2023, the company announced that Piggy Sooy seeds had achieved high levels of expression of pork protein (up to 26.6% of the total soluble protein) and had patented their technology. The company clarifies that Piggy Sooy development is set to keep moving forward completing the necessary consultation with the United States Food and Drug Administration (FDA). Moolec declares to be engaged in the consultation process with the FDA, representing the next pivotal regulatory milestone preceding the commercial availability of Piggy Sooy ingredient.